How Clinical Trials Work: A Step-by-Step Guide
Clinical trials are essential to medical progress, helping researchers test new treatments, drugs, or devices on humans before they become widely available. If you’ve ever wondered, “What is a clinical trial?”, this guide breaks down the process, including the different phases and what to expect. Understanding how clinical trials work ensures that participants and healthcare professionals are fully prepared.
Definition for Clinical Trials
First, let’s define “clinical.” The definition for clinical refers to anything related to the observation and treatment of patients, especially in a healthcare setting. In the context of a clinical trial, it means studies involving human participants, aimed at evaluating medical, surgical, or behavioral interventions. These trials are critical for determining the safety and effectiveness of new therapies before they are approved for general use.
What is a Clinical Trial?
A clinical trial is a research study that tests how well new medical approaches work in humans. These studies investigate the safety, efficacy, and side effects of treatments. They help determine if a new drug, treatment, or medical device is safe and effective for widespread use.
Clinical trials are categorized into several phases, each serving a specific purpose:
- Phase I (1): The first stage, typically involving a small group of participants, focuses on safety. Researchers aim to identify any side effects and determine the correct dosage.
- Phase II (2): This phase involves more participants and assesses the treatment’s efficacy. Researchers monitor for side effects and test if the treatment works as intended.
- Phase III (3): Large-scale trials that compare the new treatment to existing ones. This phase further assesses efficacy and monitors adverse reactions.
- Phase IV (4): Post-approval studies conducted after the treatment has been approved for public use, to monitor long-term effects.
Define Clinical Research in Trials
To define clinical research, it refers to studies aimed at improving medical knowledge by involving human participants. This can range from observing behavior to testing a new medical treatment. Clinical trials fall under clinical research and typically involve a clinical study protocol, which outlines every step of the study, including participant criteria, procedures, and timelines.
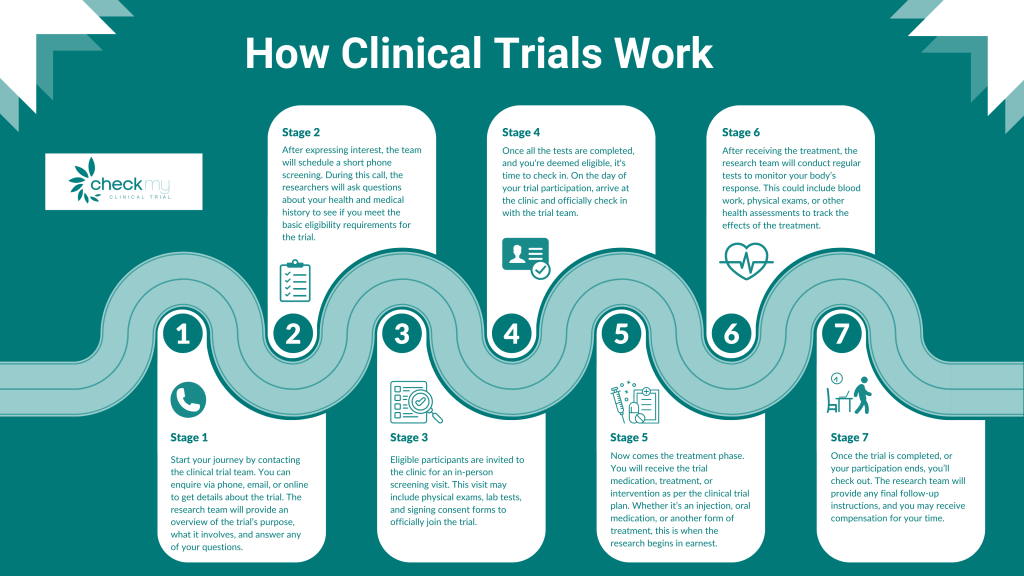
How Clinical Trials Work: The Process
Here’s an overview of the main steps involved in a clinical trial:
- Preclinical Testing: Before involving humans, the treatment undergoes preclinical trials in labs or on animals. This ensures basic safety before testing on people.
- Volunteer Screening: The trial needs specific types of participants. Researchers look for people who meet certain conditions, such as age, gender, health status, and medical history.
- Informed Consent: Participants are given all the information about the trial, including its purpose, risks, benefits, and procedures. They must sign a consent form before joining.
- Trial Execution: Participants are divided into groups (sometimes including a control group) and given the treatment or a placebo. They may receive the treatment in a clinic or hospital and will be monitored throughout.
- Data Collection: Researchers track the effects of the treatment on participants, recording data on side effects, efficacy, and overall health outcomes.
- Results and Approval: Once a trial is complete, the results are analyzed. If the treatment proves safe and effective, it may be submitted for regulatory approval, such as from the FDA or MHRA.
Why Participate in a Clinical Trial?
Participating in a clinical trial allows individuals to access new treatments that may not yet be available to the public. Some trials also offer compensation, and participants play a role in advancing medical science. However, it’s important to weigh the risks, as new treatments may have unknown side effects.
Ensuring Safety in Clinical Trials
Clinical trials follow strict safety protocols, monitored by ethics committees and regulatory authorities. Every trial must adhere to a detailed clinical study protocol that outlines the procedures for ensuring participants’ safety. Before any trial begins, it must receive approval from authorities like the FDA or MHRA to ensure it meets safety standards.
Finding a Clinical Trial
Many websites, such as ClinicalTrials.gov, provide up-to-date listings of clinical trials, including paid clinical trials in the UK. By reviewing the trial’s clinical study protocol and understanding its design, participants can make informed decisions.
This guide to clinical trials covers everything from the definition for clinical research to the process of trial participation. If you’re considering joining a clinical trial, be sure to discuss it with a healthcare provider to understand the risks and benefits fully.
Would you like to explore available trials? Visit platforms like ClinicalTrials.gov or NIHR’s Be Part of Research to find opportunities.