Frequently Asked Questions (FAQ’s)
What Are Clinical Trials?
Clinical trials are research studies performed on human volunteers to evaluate medical, surgical, or behavioral interventions. They are the primary way researchers determine whether new treatments, drugs, or procedures are safe and effective.
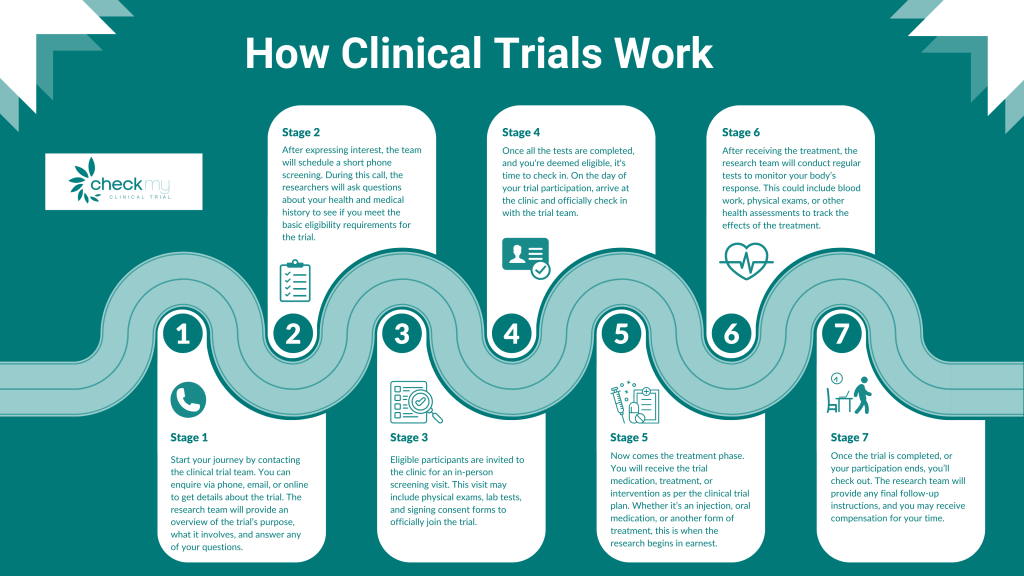
How Do Clinical Trials Work?
Clinical trials follow a pre-defined plan or protocol. Participants are given specific treatments or interventions, and their health is monitored to determine the effectiveness and safety of the intervention. Results from clinical trials are used to help guide future medical practices.
Who Can Participate in Clinical Trials?
Eligibility criteria vary from trial to trial, but generally, anyone can participate, depending on the study’s requirements. Some trials recruit healthy volunteers, while others need participants with specific health conditions.
Are Clinical Trials Safe?
Clinical trials must follow strict regulatory guidelines to protect participants. Each trial is overseen by medical professionals and ethical review boards to ensure the safety of participants. While trials may carry risks, these are carefully monitored.
What Are the Benefits of Participating in Clinical Trials?
Participants in clinical trials may gain access to new treatments, receive additional medical care, and contribute to the advancement of medical knowledge. Some trials also offer financial compensation.
Do Clinical Trials Pay Participants?
Yes, many clinical trials offer compensation to participants. Payment varies based on the length and complexity of the trial. Early-phase trials, like Phase I studies, often offer the highest compensation.
How Much Can I Earn from Clinical Trials?
Compensation varies, but participants in Phase I clinical trials can earn between £500 and £5,000 depending on the trial’s duration and risk. Longer and more invasive trials tend to pay more.
What Are the Phases of Clinical Trials?
Clinical trials are conducted in phases:
- Phase I: Tests the safety and dosage of a new treatment on a small group of healthy volunteers.
- Phase II: Focuses on the treatment’s efficacy in a larger group of people.
- Phase III: Confirms the treatment’s effectiveness with a large group and compares it to standard treatments.
- Phase IV: Conducted after the treatment is approved to monitor long-term effects.
What Should I Expect During a Clinical Trial?
Before the trial begins, participants will go through a screening process to ensure they meet the trial’s eligibility requirements. During the trial, you may be asked to take medication, undergo tests, and attend follow-up visits. Researchers will monitor your health throughout the study.
Can I Leave a Clinical Trial Once I Start?
Yes, you have the right to withdraw from a clinical trial at any time, for any reason, without any penalty. You will still receive payment for the portion of the study you completed, based on the trial’s compensation structure.
How Do I Find Clinical Trials Near Me?
You can find clinical trials by using online databases such as:
These platforms allow you to filter by location, condition, and compensation.
What Is Informed Consent in Clinical Trials?
Informed consent is the process by which participants are educated about the details of the clinical trial, including its risks and benefits. Before you join, you must sign an informed consent form that explains everything you need to know about the study.
What Are the Risks of Participating in Clinical Trials?
Risks vary depending on the type of trial. Early-phase trials might have more risks because they are testing a treatment for the first time. However, all risks are carefully outlined before you participate, and your health is closely monitored throughout the process.
What Types of Clinical Trials Are There?
There are different types of clinical trials, including:
- Treatment Trials: Test new treatments or combinations of drugs.
- Prevention Trials: Explore ways to prevent illness.
- Diagnostic Trials: Investigate new ways to diagnose conditions.
- Screening Trials: Test methods of detecting diseases.
- Quality of Life Trials: Focus on improving the quality of life for individuals with chronic illness.
Are There Clinical Trials for Healthy Volunteers?
Yes, many trials require healthy volunteers to test new treatments, especially in Phase I clinical trials. Healthy participants help determine if the treatment is safe before it’s tested on individuals with the condition being studied.
What Are the Disadvantages of Clinical Trials?
While participating in a clinical trial can offer access to new treatments, there are risks, including potential side effects and time commitments. There’s also no guarantee that the treatment will work for you.
How Do I Sign Up for Clinical Trials?
To sign up for a clinical trial, first search for available studies on websites like ClinicalTrials.gov or Be Part of Research (NIHR). Once you find a trial that matches your eligibility, follow the instructions to contact the research team, who will guide you through the application and screening process.
Can I Participate in Multiple Clinical Trials at Once?
Typically, you cannot participate in multiple clinical trials simultaneously, as treatments from one study might interfere with another. Most research centers require a “washout period” between trials to ensure your body has returned to baseline before starting another study.
What Should I Look for in a Clinical Trial?
When considering a clinical trial, review the study protocol, potential risks, compensation, the duration of the trial, and how the study might impact your daily life. Always ensure the trial has ethical approval from regulatory bodies like the FDA or MHRA.
How Long Do Clinical Trials Last?
Clinical trials can last anywhere from a few weeks to several years, depending on the phase and type of study. Early-phase trials (like Phase I) often last only a few days or weeks, while Phase III trials can span months or years with multiple follow-up appointments.
What Is a Clinical Study Protocol?
A clinical study protocol is a detailed plan that explains the purpose, methodology, and procedures of a clinical trial. It includes eligibility criteria, participant requirements, safety measures, and data collection methods, ensuring the study is conducted consistently and ethically.
Can You Make a Living From Paid Clinical Trials?
While paid clinical trials can provide substantial compensation, especially for Phase I trials, they should not be considered a long-term source of income. Compensation varies, and there are limits to how often you can participate due to safety regulations.
Are There Clinical Trials for Specific Conditions?
Yes, many clinical trials focus on specific health conditions such as cancer, diabetes, asthma, heart disease, and more. These trials test new treatments for these diseases and often recruit participants who have been diagnosed with the condition.
How Are Participants Chosen for Clinical Trials?
Participants are chosen based on the inclusion and exclusion criteria of the clinical trial. These criteria may include factors like age, gender, health condition, medical history, and current medications. Only participants who meet the criteria can enroll in the study.
What Are the Side Effects of Clinical Trials?
Potential side effects vary depending on the treatment being tested. Participants in clinical trials may experience mild to severe side effects, which are monitored closely by the research team. All known risks are disclosed during the informed consent process.
How Are Clinical Trials Monitored?
Clinical trials are monitored by the research team, ethics committees, and regulatory bodies like the FDA to ensure participants’ safety. Data is regularly reviewed, and safety protocols are in place to protect participants from harm.
What Happens After a Clinical Trial Ends?
After a clinical trial ends, researchers analyze the data collected to determine the effectiveness and safety of the treatment. Participants may be asked to attend follow-up appointments, and the trial results are often published or submitted to regulatory authorities for approval.
Can Clinical Trials Help Cure Diseases?
While clinical trials are essential in developing new treatments, they don’t guarantee a cure. Clinical trials are designed to test the safety and efficacy of new treatments, but the outcome of a trial may not always lead to a cure.
Can I Participate in Clinical Trials If I Have Pre-Existing Conditions?
Yes, many trials target individuals with specific medical conditions. However, it’s important to find trials that match your medical history and current health status. Certain conditions may exclude you from certain trials due to safety risks.