The Role of Clinical Trials in Pandemic Response: COVID-19, Swine Flu, and More
Clinical trials form the backbone of medical research, ensuring that vaccines and treatments are safe and effective before they reach the public. During pandemics like COVID-19, swine flu, Ebola, and SARS, clinical trials have been the key to fast, life-saving vaccine developments. In this blog, we’ll dive deep into each of these outbreaks, examine the various COVID-19 vaccines, explore vaccine costs, side effects, and even the financial outcomes for pharmaceutical companies. This comprehensive guide will answer common questions about COVID-19 and beyond, offering a thorough look at how vaccines change the world.
1. The Basics of COVID-19: Origins, Causes, and Core Questions
How did COVID-19 start?
COVID-19 is widely believed to have originated from a wildlife market in Wuhan, China, in late 2019. The virus, SARS-CoV-2, likely jumped from an animal species to humans, a transmission type known as zoonosis. High human mobility and global travel then spread the virus worldwide, leading to the pandemic.
What is the cause of the COVID-19 virus?
The SARS-CoV-2 virus, part of the larger coronavirus family, is the cause of COVID-19. Coronaviruses are named for the crown-like spikes on their surface (corona meaning crown in Latin). SARS-CoV-2 is more transmissible than its coronavirus cousins, like SARS-CoV or MERS-CoV, which partly explains its rapid spread.
What does COVID stand for?
COVID stands for “Coronavirus Disease 2019,” referring to the year the disease was first identified.
What were the major problems with the COVID-19 pandemic?
The pandemic led to overloaded healthcare systems, economic downturns, mental health crises, and supply chain disruptions. High infection rates and severe cases overwhelmed hospitals, causing shortages of medical supplies and beds. Social isolation measures, while necessary, had a profound impact on mental health worldwide, highlighting the many indirect effects of a pandemic.
2. Understanding COVID-19 Vaccines: Types, Development, and Effectiveness
What are the different types of COVID-19 vaccines?
COVID-19 vaccines use various technologies to induce immunity, each with unique approaches:
- mRNA Vaccines: Pfizer-BioNTech and Moderna vaccines use messenger RNA (mRNA) to instruct cells to produce the SARS-CoV-2 spike protein, prompting an immune response.
- Viral Vector Vaccines: AstraZeneca and Johnson & Johnson use a weakened virus (not SARS-CoV-2) to deliver the spike protein.
- Protein Subunit Vaccines: Novavax, for example, includes only harmless virus protein fragments, rather than the whole virus, to stimulate an immune response.
- Inactivated or Live Attenuated Vaccines: Sinopharm and Sinovac developed vaccines using inactivated or weakened SARS-CoV-2 virus particles.
What is the current COVID vaccine in the UK?
As of 2024, the UK continues to use updated mRNA vaccines from Pfizer-BioNTech and Moderna, designed to address the latest variants. Booster shots are recommended annually, similar to the flu vaccine, particularly for at-risk populations.
How was the COVID vaccine developed so quickly?
COVID-19 vaccines were developed quickly due to global collaboration, cutting-edge mRNA technology, and substantial government funding. Traditional vaccine trials can take years, but during COVID-19, regulatory bodies like the FDA and EMA used rolling reviews, allowing them to review data as it was generated.
How effective are COVID-19 vaccines?
Initial mRNA vaccines (Pfizer and Moderna) were about 95% effective at preventing symptomatic cases in early trials. Viral vector vaccines like AstraZeneca demonstrated around 70-80% effectiveness, though effectiveness can vary based on the viral variant.
3. The Clinical Trial Process for COVID-19 Vaccines: A New Era
Are there clinical trials for COVID-19?
Yes, COVID-19 vaccines went through extensive clinical trials worldwide. These trials enrolled tens of thousands of volunteers, testing vaccines in multiple phases to ensure safety and efficacy.
How did COVID-19 change clinical trials?
COVID-19 accelerated the clinical trial process and introduced “master protocols” where multiple vaccines could be tested under one framework. This was faster and more efficient, allowing resources to be used flexibly. Additionally, Phase 3 trials were scaled up globally, involving unprecedented numbers of participants, giving a more robust dataset.
How long did the COVID vaccine trials last?
Phase 1 trials for COVID-19 vaccines began as early as March 2020, with Phase 3 results published by November 2020. Although the trials lasted only a few months, real-world data from millions of people continues to provide safety and efficacy updates.
What is the best treatment for COVID-19?
Currently, antiviral drugs (like Paxlovid) and monoclonal antibodies are the main treatments to prevent severe COVID-19 cases. Vaccination remains the primary preventative measure, but these treatments offer additional support, especially for high-risk individuals.
4. Vaccine Types, Boosters, and Global Distribution
What are the 4 types of COVID-19 vaccines?
The four main types include mRNA, viral vector, protein subunit, and inactivated/live attenuated vaccines. Each type has unique benefits and production methods, catering to different needs and infrastructure capabilities globally.
What vaccines were used in the UK?
The UK’s vaccine portfolio included Pfizer-BioNTech, Moderna, AstraZeneca, and Johnson & Johnson. By offering multiple options, the UK could vaccinate a broader demographic quickly.
What is the latest vaccine for COVID?
As of 2024, variant-specific vaccines targeting strains like Omicron have been developed, with mRNA vaccines being updated to enhance protection against these variants.
How often should I have a COVID vaccine?
The current recommendation is for annual boosters, particularly for high-risk groups like the elderly and those with underlying health conditions.
5. Names and Types of COVID-19 Vaccines Across Countries
What are the names of COVID vaccines in the UK?
Pfizer-BioNTech (Comirnaty), Moderna (Spikevax), and AstraZeneca (Vaxzevria) are the main COVID-19 vaccines administered in the UK, with occasional doses of Johnson & Johnson for single-dose requirements.
What are the names of the COVID-19 vaccines in the Philippines?
In the Philippines, vaccines like Pfizer-BioNTech, Moderna, AstraZeneca, Sinovac, and Sputnik V were commonly used.
What are the 7 types of vaccines?
While not all types are used for COVID-19, the main vaccine technologies include mRNA, viral vector, protein subunit, inactivated virus, live attenuated virus, virus-like particles, and DNA-based vaccines.
6. Side Effects and Risks of COVID-19 Vaccines
What are the side effects of the COVID vaccine?
The most common side effects include soreness at the injection site, fatigue, fever, and headache. Some people experience more severe side effects, like myocarditis (inflammation of the heart muscle), primarily observed in younger males with mRNA vaccines.
What are the risks of vaccines?
Vaccines are generally safe, but rare risks exist, such as allergic reactions (anaphylaxis) and, in some cases, blood clotting issues with the AstraZeneca vaccine. Monitoring systems are in place to manage these risks effectively.
What are the bad reactions to vaccines?
Severe reactions, though rare, include anaphylaxis, myocarditis, and Guillain-Barré syndrome. However, these risks are very low, especially compared to the protection vaccines provide against severe illness.
What are the side effects of the new COVID vaccine in 2024?
The latest vaccines have similar side effects to previous ones—soreness, fatigue, and mild fever. Adjustments in formulation have minimized more severe reactions, improving overall safety.
7. Financial Costs and Revenues: Development, Pricing, and Profits
What was the cost to develop the COVID-19 vaccine?
Developing the COVID-19 vaccine was a billion-dollar endeavor. Pfizer-BioNTech and Moderna each spent over $1 billion, with significant funding from government sources to offset risks and ensure speedy production.
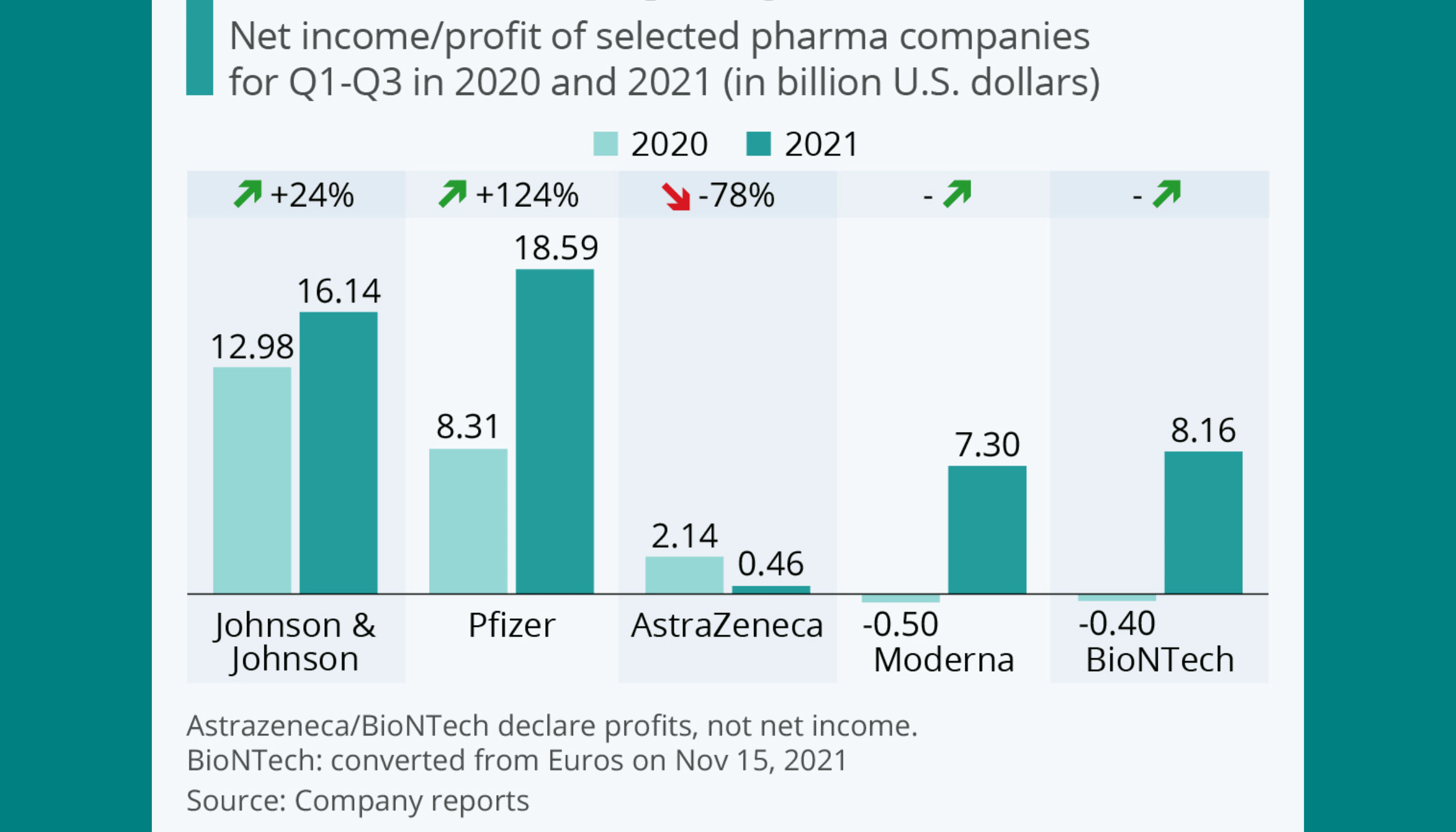
How much revenue did companies make from COVID-19 vaccines?
The revenue from COVID-19 vaccines was substantial:
- Pfizer-BioNTech: Generated over $36 billion in 2021 alone.
- Moderna: Earned around $18 billion from its vaccine in 2021.
- Johnson & Johnson: Reported approximately $2.39 billion from vaccine sales.
Impact on Healthcare and Economic Savings
These vaccines not only saved lives but also reduced the burden on healthcare systems. The cost savings from preventing hospitalisations and deaths were enormous, helping to stabilize economies.
The Lasting Impact of Clinical Trials on Global Health
Through these clinical trials, the world has seen how crucial vaccines are in protecting populations and preventing healthcare systems from being overwhelmed. From COVID-19 to H1N1 and Ebola, clinical trials ensure that vaccines are safe, effective, and accessible. By answering questions about vaccine types, side effects, and economic impacts, this blog highlights the indispensable role of trials in global health.
Leave a comment