What is a Clinical Trial? Simple Definition, UK Process & Why Participate
When discussing medical research, you’ll often hear the term clinical trial. These trials are critical in developing new treatments, medicines, and even preventative measures. But what exactly is a clinical trial, how do they work, and why do people participate? find out more (how clinical trials work).
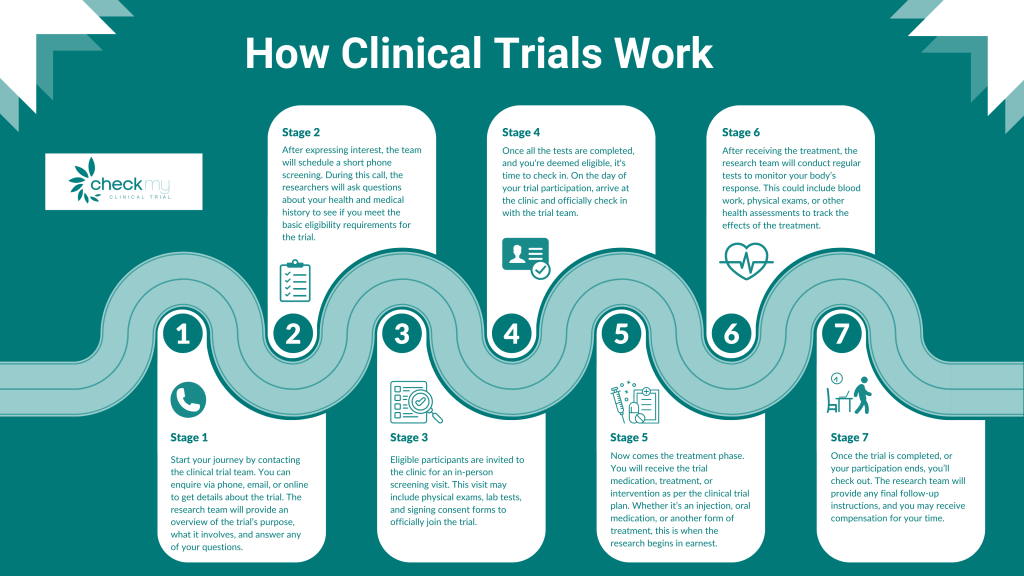
In this blog, we’ll break down the key components of a clinical trial, focusing on what happens during trials, how they’re conducted in the UK, and the reasons why someone would volunteer. If you’ve ever wondered about clinical trials or are considering participating, this guide is for you.
What is a Simple Definition of a Clinical Trial?
Let’s start with the basics: a clinical trial is a carefully designed research study involving human participants. It tests medical interventions such as new drugs, surgical procedures, or devices. The ultimate goal is to evaluate the safety and effectiveness of these treatments before they are made available to the public.
In simpler terms, clinical trials help turn scientific discoveries into treatments that can be used by healthcare providers to improve patient outcomes. Without clinical trials, we wouldn’t have vaccines, life-saving drugs, or advanced medical technologies.
What Happens in Clinical Trials?
Every clinical trial follows a structured process designed to ensure participant safety and gather accurate data. Clinical trials are usually divided into four phases. Each phase has a distinct purpose, but all focus on determining the best possible treatment for a specific medical condition.

Here’s an overview of what happens in each phase:
Phase 1: Initial Safety Testing
Phase 1 focuses on the safety of a new treatment. Researchers administer the treatment to a small group of healthy volunteers or patients to evaluate its safety, determine a safe dosage range, and identify any potential side effects.
Phase 2: Effectiveness and Side Effects
Once the treatment has passed Phase 1, it moves to Phase 2. This phase involves a larger group of participants and aims to assess the effectiveness of the treatment and further evaluate its safety.
Phase 3: Comparison and Monitoring
In Phase 3, the treatment is given to even larger groups of people to confirm its effectiveness, monitor side effects, and compare it with standard or placebo treatments. This phase provides the most comprehensive data before the treatment can be approved by regulatory authorities.
Phase 4: Long-Term Effects
Once a treatment is approved and in use, Phase 4 trials begin. These trials track the long-term effectiveness and side effects of the treatment in large populations. Phase 4 ensures the treatment remains safe and beneficial over time.
Each phase plays a vital role in ensuring treatments are safe, effective, and suitable for public use.
What is a Clinical Trial in the UK?
Clinical trials in the UK follow rigorous guidelines set by various regulatory bodies to ensure participant safety and reliable results. The UK is known for its high standards in clinical research, making it a trusted location for these studies.
Who Regulates Clinical Trials in the UK?
In the UK, clinical trials are overseen by several authorities, including:

- Medicines and Healthcare products Regulatory Agency (MHRA): Ensures that the trials meet safety and ethical standards before they begin.
- National Institute for Health and Care Research (NIHR): Funds research and provides infrastructure support.
- Research Ethics Committees (REC): Review the ethical aspects of each trial to protect participants.
These organizations ensure that all clinical trials in the UK are conducted with participant well-being in mind. Before any trial starts, researchers must submit their study for approval, ensuring that every aspect of the trial has been rigorously reviewed.
Where are Clinical Trials Conducted in the UK?
Clinical trials in the UK are often conducted through:
- National Health Service (NHS): Many trials take place in NHS hospitals and clinics, ensuring access to a diverse population.
- Universities: Academic institutions often collaborate with healthcare providers and pharmaceutical companies to conduct cutting-edge research.
- Private Research Organizations: These organizations conduct trials independently or on behalf of pharmaceutical companies.
The NHS and university partnerships often give trials a unique advantage in terms of diversity, access to specialists, and ethical oversight.
Why Would Someone Do a Clinical Trial?
People participate in clinical trials for a range of reasons, from personal benefits to altruistic motivations. Here are a few common reasons why someone might choose to participate:
Access to New Treatments
One of the main reasons people join clinical trials is to access new treatments before they are widely available. This is particularly true for patients with conditions that have limited treatment options. For example, a cancer patient might join a trial to try a cutting-edge therapy that’s not yet available through traditional healthcare channels.
High-Quality Medical Care
Participants in clinical trials often receive top-quality medical attention. The trial process usually includes regular check-ups, monitoring, and thorough health assessments, which can be especially beneficial for individuals with chronic conditions.
Contributing to Science
Many participants volunteer because they want to contribute to scientific progress. By taking part, they help researchers develop new treatments that could benefit future patients.
Financial Compensation
While not the primary reason for most participants, some clinical trials offer financial compensation or reimburse expenses like travel. This can be a motivating factor, especially for healthy volunteers participating in early-stage trials. (How much do clinical trials pay?)
Important Considerations Before Joining a Clinical Trial
Before deciding to participate, it’s important to consider a few factors:
- Informed Consent: Participants must be fully informed about the potential risks and benefits before they agree to take part. You can read more about the importance of informed consent on this NHS page about clinical trials.
- Possible Risks: Every clinical trial carries some level of risk. This can range from minor side effects to more serious outcomes depending on the treatment being tested. It’s important to discuss these risks with the trial organizers and your healthcare provider.
- Time Commitment: Some trials require a significant time investment, including multiple visits, regular check-ups, and follow-up appointments. Make sure you understand the commitment before joining.
How to Find Clinical Trials in the UK
Finding a clinical trial that fits your health condition or interests can be done through various platforms. The NHS provides a comprehensive list of ongoing trials through their Be Part of Research platform. Additionally, the UK ClinicalTrials Gateway is an excellent resource for finding detailed information on trials open to participants across the UK. Use our top clinical trial compare page (here)

Leave a comment